Introduction
As most people know, chemistry evolved from alchemy, just as astronomy evolved from star gazing and from astrology. Alchemists tried to produce gold from less expensive elements. They failed and this led to the realization that chemical elements are immutable. The idea of immutability of elements, confirmed by all chemical experiments, is no longer as absolute as it used to be; we know that nuclear reactions often result in transmutation of elements. But this happens only when kinetic energies of subatomic particles (neutrons, protons and their aggregates) exceed those encountered in ordinary chemical experiments. An observed transmutation of elements at low temperature, if confirmed, would indicate that subatomic particles are somehow accelerated to unusually high energies.
Suppose that the potential difference between two electrodes is 100 volts. It could be used to accelerate protons to an energy of 10 eV. This is not sufficient to overcome an electrical repulsion of atomic nuclei. But suppose that a proton is attached to a cloud of a million electrons. (Speculations about the possible stability of such a cloud are described in Appendix 2.) A proton attached to the cloud, in traveling toward the positive electrode, could gain kinetic energy of ten million eV. This would be sufficient to penetrate a nucleus and cause a nuclear reaction. The purpose of this essay is to summarize two articles about experimental evidence of nuclear transmutations occurring in simple tabletop experiments. The authors of the articles, Hal Fox and Shangxian Jin, invited me to work with them and I will summarize what I know about their work. I plan use this essay as a basis for writing about the outcome of my ten-day-long visit to their private laboratory. This summary is limited to experimental facts; comments and tentative speculations are to be found in appendices. The articles were published in the Journal of New Energy (1,2); Hal Fox, from Salt Lake City, is the editor of that journal.
Experimental Setup
The setup, called LENT-1 reactor, was essentially a vessel with two electrodes made of oxidized zirconium. It was partially filled with 25 cc of distilled water in which various amounts of thorium nitrate (between 0.1 and 0.5 grams) were dissolved. The vessel was hermetically sealed to withstand high pressures (tens of atmospheres) at high temperatures (up to 500 F). The electrodes were connected to secondary winding of a power transformer (up to 2 kVA). The typical current flowing through the electrolyte was 5A while the effective a.c. voltage was typically less than 100V. The potential differences across the electrolyte were very small in comparison with potential differences along the very thin layers of zirconium oxide covering the electrodes. This fact seems to be significant, as elaborated in Appendix 2.
Thorium nitrate was chosen because it is highly soluble compound containing several radioactive nuclides (232Th in equilibrium with its daughters). The main purpose of the experiments was to show that the initial radioactivity can be reduced very significantly during short time intervals, most often 30 minutes. Let me mention that numerous attempts to reduce radioactivity, shortly after radium was discovered by Marie Curie, were made but they were not successful. According to (3): “High and low temperatures were tried, strong electric and magnetic fields were applied, tremendous pressures, and the strongest possible chemical reagents were put to use, but not a single one of the most powerful weapons of the physical laboratories affected the radiation of energy by radium.” In that context the findings of Fox and Jin are very puzzling. At present I accept their data but during the experiments my position will be different; I will do everything possible to question their findings. But I will be open-minded; I will change my skeptical attitude if we fail to contradict the data.
Summary of experimental results
The authors write that it took them three years to develop a procedure for the effective transmutation in the LENT-1 reactor. Over a dozen successful experiments were performed. The method used consisted of comparing radioactivity “before” the current was turned on with radioactivity “after” the current was turned off, 30 minutes later.
1) A Geiger counter measuring radioactivity of the electrolyte showed practically nothing but the background after the processing. The level of a constant radioactivity before the experiment was “considerably above the background.”
2) The same counter was used to measure the radioactivity from the surface of electrodes. The electrodes were not radioactive initially but became radioactive after 30 minutes of processing. The most remarkable was that the surface radioactivity was not constant, it decreased, more or less exponentially to a constant level (about 90 counts per minute) after 200 hours. This was interpreted as an indication that relatively short-living radioactive nuclides were formed through nuclear reactions.
3) A mass spectroscopic analysis of electrolyte, performed in a specialized laboratory, showed that 95% of thorium was lost. This was consistent with what has been inferred from the Geiger counter data (see point 1 above).
4) The second article of Fox and Jin focuses on the use of two gamma ray spectrometers, one using a pure Ge detector. The mass spectrometric analysis showed that the amount of Th in the electrolyte was reduced from 4399 ppm to 9.5 ppm. This confirmed findings reported in the first article.
5) The below shows the cutaway view of the vessel and the location of the Ge detector. The gamma ray spectra (actually shown in the publication) were obtained during one hour interval immediately after the end of processing. The vessel was not open and gamma rays had to penetrate the 1 cm wall of zirconium.
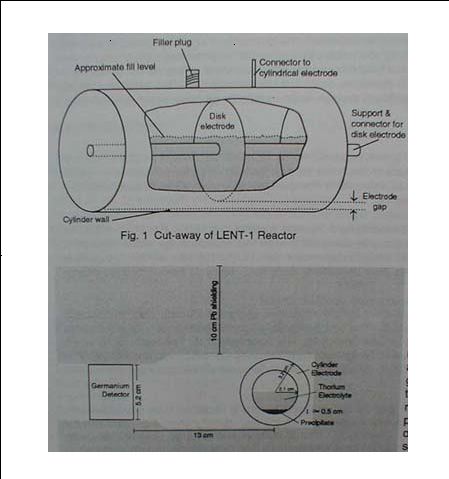
Figure 1.
The setup shown to me was slightly different. It was essentially a zirconium cup whose central electrode (a zirconium rod) was supported by a covering teflon plate.
Intensive underwater arcing and sparking accompany the flow of current.
= = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = =
As illustrated below, the radioactivity from the entire setup (based on four well known gamma ray peaks due to daughters of thorium) was reduced by approximately 50%. This is five times more that could possibly be attributed to a change in the distribution of radioactivity (concentration in the precipitate).