INTRODUCTION
Cold fusion (LENR) has been difficult to duplicate and many proposed explanations have not helped to solve this problem. I suggest the difficulty is caused by an erroneous assumption. LENR reactions obviously do not occur everywhere in nature. Painful experience has shown that they occur only in special and difficult to create materials, the so-called NUCLEAR ACTIVE ENVIRONMENT. In the past, people have assumed that the NAE is in the bulk of a Pons-Fleischmann (P-F) palladium cathode. The surface of the cathode is assumed to be inert and only serves to modify the composition of the underlying beta-PdD. In contrast, I’m proposing that the surface layer itself is the NAE.
The concept of NAE is difficult for people trained in physics to accept because phenomenon studied by physics, particularly nuclear physics, are independent of chemical form. For example, when sufficient plutonium is brought together in one place, the resulting nuclear reaction does not depend on whether the material is the carbide, nitride or oxide. However, in the case of LENR the environment is very important. When the proper environment is achieved, the nuclear reaction occurs spontaneously, with no additional effort required. A person does not have to understand the mechanism of the process. Unfortunately, most theories have focused on the mechanism rather than on the environment. To make matters worse, the mechanisms are applied to ideal, imagined materials, not to those existing in the real world. As a result, little useful progress has been achieved.
DISCUSSION
To discover the properties of the NAE, it’s location on the cathode must be determined. The following observations suggest that it is located in the surface region of the cathode.
1. Helium generated by LENR is found in the surrounding gas rather than in the palladium deuteride.
When helium is placed in palladium using decay of dissolved tritium, the resulting
3He can be removed only by heating near the melting point [1]. Only helium located
near the surface is able to leave the material without this treatment. Therefore, the
observed anomalous 4He must have been generated very near the surface of a P-F
cathode to be observed in the surrounding gas.
2. Anomalous tritium is detected in the electrolyte rather than in evolving gas.
Tritium that is known to be present within the cathode can be displaced by deuterium
during electrolysis. This tritium is found to leave with the evolving deuterium gas [2].
However, tritium produced during a cold fusion reaction is found in the electrolyte,
provided a recombining catalyst is not present in the cell. Tritium is able to enter the
electrolyte as T+ because it is able to exchange with D+ at the cathode surface before
it has a chance to form DT molecules, which would leave as gas.
3. Anomalous heat is observed to be generated on the cathode surface.
Szpak et al. [3, 4], using an IR camera, observed hot spots that turned on and off in
rapid secession on an active cathode . The generated energy was claimed to come
from just below the surface.
4. Transmutation products are located only in the surface region.
A number of studies have detected transmutation products located within the surface
region of P-F cathodes. These elements do not exist within the bulk material[5-9].
5. Melted regions are observed on the cathode surface.
Microscopic examination of the surface shows many melted regions after anomalous
energy has been generated[10].
6. Large amounts of anomalous energy can be generated using thin films on an inert
substrate.
Significant anomalous energy has been generated using very thin films of
material[11-13]. If a large amount of palladium were required, as would be present
in bulk material, these thin films would have produced very little anomalous energy.
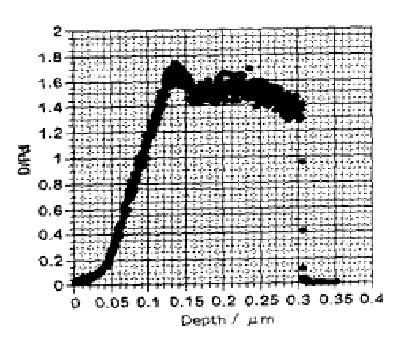
FIGURE 1. Deuterium concentration in Pd after electrolysis based on ERDA. [14] Some
deloading from the surface is evident.
Next, the deuterium content of the NAE needs to be discovered. Unfortunately, this
property is difficult to measure. Reported values are always based on the average composition, not
the composition of the surface. Two efforts to measure the composition near the surface are shown
in Figs. 1 and 2. The D/Pd ratio of the NAE will be greater than these values.
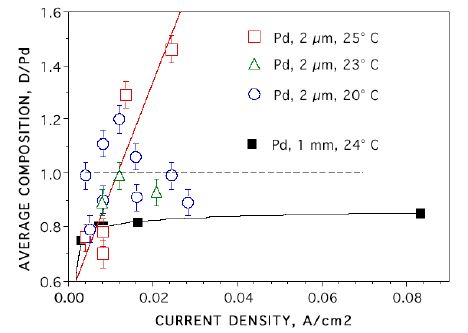
FIGURE 2. Deuterium content in Pd films plated on Pt after electrolysis based on orphaned oxygen. [15] Film thickness and temperature are indicated.
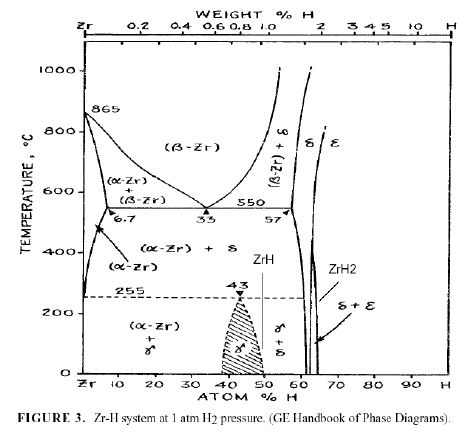
These measurements indicate that the NAE has a composition in excess of PdD1.7. The beta phase has a limiting composition of PdD1.0. Therefore, the NAE can not be beta-PdD. Of course, some people will argue that the additional D+ goes into tetrahedral sites. However, no evidence exists for occupancy of this site. In fact, if this site were occupied, a change in crystal structure would be expected as experienced by hydrides formed by other metals. For example, other metals form hydrides having a limiting composition of MH2, in addition to MH. An example is shown in Fig. 3 for the Zr-H system. The ZrH2 structure is fcc tetragonal, as would be expected if D+ occupied both tetrahedral and octahedral sites and had an ordered arrangement. If tetrahedral sites were occupied, it is safe to conclude that they would be present in PdD2 not in beta-PdD. Also, such structures have the potential for the presence of deuterium dimers. Besides having a very high D/Pd ratio, the surface also contains a high concentration of Li, Pt and Si, along with many other elements[8, 16, 17]. Consequently, the surface is a complex mixture of many elements with a very high D/M ratio and an unknown structure.
What other characteristics might the surface layer have? LENR has been produced from finely divided palladium using palladium-black [18, 19] or fine powder attached to charcoal[20]. Even microparticles of other substances imbedded in palladium are found to be active[21]. Examinations of cathode surfaces show the presence of similar microstructures as a result of the electrodeposition process. In addition, successful thin films, as mentioned previously, also contain this type of microstructure. Apparently, structures in the nanometer range of size are frequently associated with anomalous energy production.
CONCLUSION
1. Pure beta-PdD, regardless of its deuterium content, is not the environment in which LENR occurs during the Pons-Fleischmann effect. In addition, the complex alloy in which the effect occurs in this case apparently requires a very high deuterium content.
2. Nearly pure beta-PdD, when it is used as small particles, appears to be active at a relatively low deuterium content.
3. LENR requires nanosized particles and can involve various complex materials, not just palladium.
4. Most theories, mechanisms, and explanations are inadequate because they have not taken these
factors into account.
References
1. Camp, W.J., Helium Detrapping and Release from Metal Tritides. J. Vac. Sci. Technol.,
1977. 14: p. 514.
2. Storms, E. and C. Talcott-Storms, The effect of hydriding on the physical structure of
palladium and on the release of contained tritium. Fusion Technol., 1991. 20: p. 246.
3. Mosier-Boss, P.A. and S. Szpak, The Pd/(n)H system: transport processes and
development of thermal instabilities. Nuovo Cimento A, 1999. 112: p. 577.
4. Szpak, S., P.A. Mosier-Boss, and M.H. Miles, Calorimetry of the Pd+D codeposition.
Fusion Technol., 1999. 36: p. 234.
5. Dash, J. and S. Miguet, Microanalysis of Pd Cathodes after Electrolysis in Aqueous Acids.
J. New Energy, 1996. 1(1): p. 23.
6. Miley, G.H., et al. Quantitative observations of transmutation products occuring in thinfilm
coated microspheres during electrolysis. in Sixth International Conference on Cold
Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido, Japan: New
Energy and Industrial Technology Development Organization, Tokyo Institute of
Technology, Tokyo, Japan.
7. Iwamura, Y., et al. Detection of Anomalous Elements, X-ray and Excess Heat Induced by
Continous Diffusion of Deuterium Through Multi-layer Cathode (Pd/CaO/Pd). in The
Seventh International Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO,
Inc., Salt Lake City, UT.
8. Bockris, J.O.M. and Z. Minevski, Two zones of "Impurities" observed after prolonged
electrolysis of deuterium on palladium. Infinite Energy, 1996. 1(5/6): p. 67.
9. Ohmori, T., et al., Transmutation in the electrolysis of lightwater - excess energy and iron
production in a gold electrode. Fusion Technol., 1997. 31: p. 210.
10. Silver, D.S., J. Dash, and P.S. Keefe, Surface topography of a palladium cathode after
electrolysis in heavy water. Fusion Technol., 1993. 24: p. 423.
11. Miley, G.H., et al. Multilayer Thin Film Electrodes for Cold Fusion. in Third International
Conference on Cold Fusion, "Frontiers of Cold Fusion". 1992. Nagoya Japan: Universal
Academy Press, Inc., Tokyo, Japan.
12. Storms, E., A critical evaluation of the Pons-Fleischmann effect: Part 2. Infinite Energy,
2000. 6(32): p. 52.
13. Bush, R.T. and R.D. Eagleton. A Calorimetric Study of the Excess Heat Effect in Thin
Films of Palladium. in Second Annual Conference on Cold Fusion, "The Science of Cold
Fusion". 1991. Como, Italy: Societa Italiana di Fisica, Bologna, Italy.
14. Oya, Y., et al. Material Conditions to Replicate the Generation of Excess Energy and the
Emission of Excess Neutrons. in The Seventh International Conference on Cold Fusion.
1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
15. Storms, E. Relationship Between Open-Circuit-Voltage and Heat Production in a Pons-
Fleischmann Cell. in The Seventh International Conference on Cold Fusion. 1998.
Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
16. Hagans, P.L., D.D. Dominguez, and M.A. Imam. Surface composition of Pd cathodes. in
Sixth International Conference on Cold Fusion,Progress in New Hydrogen Energy. 1996.
Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology Development
Organization, Tokyo Institute of Technology, Tokyo, Japan.
17. Asami, N., et al. Material Behaviour of Highly Deuterium Loaded Palladium by
Electrolysis. in Sixth International Conference on Cold Fusion,Progress in New Hydrogen
Energy. 1996. Lake Toya, Hokkaido, Japan: New Energy and Industrial Technology
Development Organization, Tokyo Institute of Technology, Tokyo, Japan.
18. Arata, Y. and C. Zhang, Presence of helium (4/2He, 3/2He) confirmed in deuterated Pdblack
by the "vi-effect" in a "closed QMS" environment. Proc. Japan. Acad. B, 1997. 73: p. 62.
19. Arata, Y. and Y.C. Zhang. Definite Difference amoung [DS-D2O], [DS-H2O] and [Bulk-
D2O] Cells in the Deuterization and Deuterium-reaction. in 8th International Conference on
Cold Fusion. 2000. Lerici (La Spezia), Italy: Italian Physical Society, Bologna, Italy.
20. Case, L.C. Catalytic Fusion of Deuterium into Helium-4. in The Seventh International
Conference on Cold Fusion. 1998. Vancouver, Canada: ENECO, Inc., Salt Lake City, UT.
21. Storms, E., Ways to Initiate a Nuclear Reaction in Solid Environments. Infinite Energy,
2002. 8(45): p. 45.