Click to see the list of links
252 ) A student-oriented project
Ludwik Kowalski (8/28/05)
Department of Mathematical Sciences
Montclair State University, Upper Montclair, NJ, 07043
Introduction:
1) Suppose that an immersion heater is inserted into a beaker with water. How should thermal energy generated depend on the amount of electric energy used?
The answer to this question was provided by James Joule -- one joule of heat for one joule of electric energy. In a well insulated container (to minimize
losses due to convection) the temperature would rise linearly if the electric power remained constant, provided no boiling takes place.
2) And, at the boiling temperature, one would expect water to be evaporated at the rate proportional to the wattage of the heater. The latent heat of water
evaporation, under normal atmospheric pressure, is 2260 J per gram. The same is expected to be true when a small amount of salt is dissolved in distilled
water, to increase its electric conductivity. Salty water, by the way, is called electrolyte. Thus the maximum possible amount of water one can evaporate in
5 minutes, when the heating rate is 450 W, is 59.73 grams. In reality the amount of evaporated water would always be smaller, as illustrated in Figure 1
below, because some thermal energy is always lost via conduction, convection and radiation. That figure, was copied from a paper sent to me recently by
Pierre Clauzon, a French scientist from the Laboratoire d'Electrochimie Industrielle at Conservatoire National des Arts et Métiers, Paris.
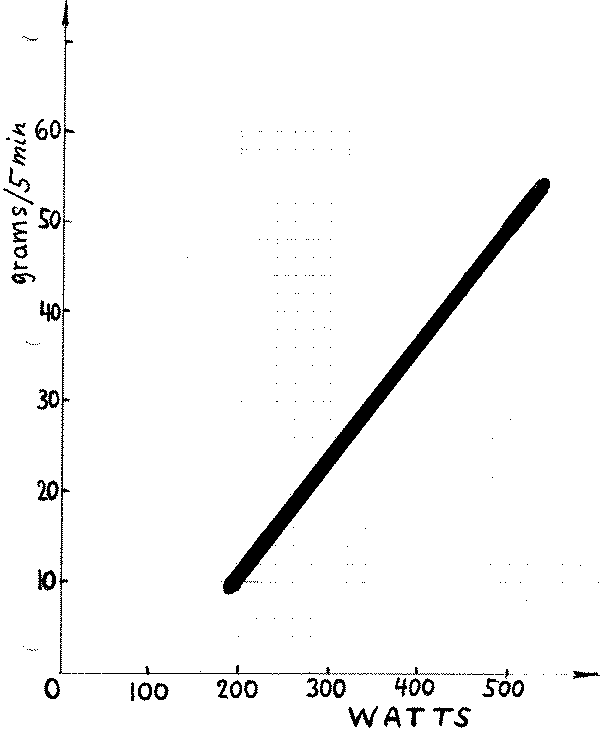
Figure 1
3) There is nothing unusual or abnormal about the black region (thick line) of that figure; it shows where a large number of experimental data points were located. The line thickness gives some idea about uncertainties of individual measurements. But the title of the paper was “Abnormal excess heat observed during Mizuno-type experiment.” Abnormalities were observed when the electrolyte was heated by a different method. Instead of passing the electric current through an ohmic heater the authors of the paper passed it through the electrolyte itself. By doing this they discovered, for example, that to evaporate 38 grams of water in 5 minutes with an ohmic resistor required the heating power of 410 W while only 300 W was required to evaporate the same amount of water in 5 minutes when the current is flowing through the electrolyte. The data point (38,300), as you can verify, is far above the black region in Figure 1. Abnormal experimental data are said to be highly reproducible.
4) How can it be? The amount of evaporated water should be the same no matter how a given amount of thermal energy is delivered to it, provided the same fraction of thermal energy is used to evaporate water. That is the dilemma. And that is why I think that attempts to replicate the experiment are worth the effort. The purpose of this note is to start a cooperative project in which several teachers would be performing essentially the same experiment at the same time. Then we can try to publish a paper that either confirms or contradicts the claimed results. If we can finish this before November 20 then I would be able to deliver this collective paper at an international conference. Schools are likely to be proud of such contributions, especially when their students are also involved.
5) Yes, I know that some might consider this suggestion ridiculous. Why bother if we already know that the claim makes no sense? Let me answer this question. By performing this experiment, preferably with a group of students, we will have a chance to practice scientific methodology. How often do we find a situation in which students can deal, in a meaningful way, with a real controversy? Here we have an opportunity to: (a) promote critical thinking, (b) focus on careful experimentation, propagation of errors, etc. and (c) possibly, if not probably, confirm a scientific discovery. The French paper has not yet been published but the authors gave me permission to write about its content, and to initiate collective students-oriented attempts to replicate the results.
6) My interpretation of such results was that some chemical reactions, taking place in the cell with the electrolyte, are producing heat in addition to electrically delivered thermal energy. In fact, I know that the tungsten rod, through which electrons enter into the solution, is partially consumed during the electrolysis. In an e-mail message to Pierre I wrote that production of heat through chemical reactions would not be an abnormal process. Here is his reply: “I know that my friends at EDF (Electricité De France) have carefully studied these problems and their answers are negative. You cannot explain the abnormal excess energy by chemical reactions with the tungsten rod. I will ask my friend Olivier to maybe elaborate more in this subject. He is a real chemist, not me !” Well, for the time being I will accept this answer; I asked for a reference to a published or unpublished report. I will insert the reference here, as soon as it arrives.
INSERTED ON 9/7/05: THE PAPER THAT PROMPTED ME IS NOW ACCESSIBLE OVER THE INTERNET:
http://lenr-canr.org/acrobat/FauvarqueJabnormalex.pdf
Experimental setups
7) Participants in this research will receive the French paper; do not be scare, it is in English. My anticipated setup is shown in Figure 2 below. It will be a graduated beaker (capacity close to 2 liters) containing a solution of the potassium carbonate salt, K2CO3, in distilled water. The concentration of the electrolyte will be 20 grams per liter (0.2 M). The electrodes (tungsten cathode and platinum anode) will have to be somehow mounted on the beaker. An ohmic heater, not shown in Figure 2, will also be mounted on the scale.
8) The first step would be to collect data with the ohmic heater. This will show where the normal data points are located (see Figure 1) for my experimental setup. Each experimental setup will probably produce a slightly different line, depending on the size of the beaker, on the lab temperature, etc. But once established, this line becomes a reference for the so-called “abnormal” results. Naturally, one has to calibrate the ohmic heater connected, for example, to a variac. Likewise one will need a high voltage power supply able to deliver up to 2 A and 600V. A sensitive scale, to support the setup, as in Figure 2, will also be needed. Note that the W cathode is placed in a glass tube; only about 1.5 cm of the cathode is below the lower end of that tube.
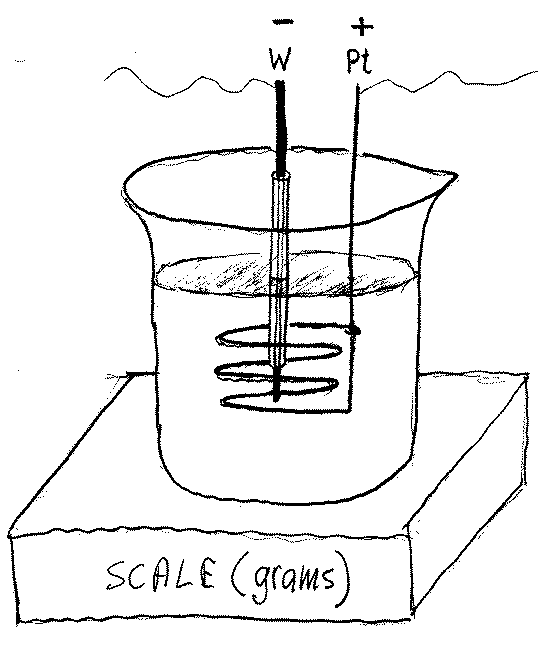
Figure 2
9) The Pt anode will be a cylinder-like spiral wire situated 4 cm from the cathode, as in French experiments. The cathode will be a commercially available tungsten rod used for welding. The water level would have to be the same at the beginning of each five-minute-long test. I do not think that replacing evaporated water during a test (from a container mounted on the scale) is worth the effort because changes in the level of the electrolyte are relatively small. On the other hand, I anticipate problems with water condensation on the walls of the beaker, and with possible splashing, abobe 200 W. Each of these two effects, if not accounted for, can contribute to an apparent excess heat.
10) I know that spectacular underwater glow discharges will be seen around the cathode. That will be associated with significant fluctuations of electric current. But the glow discharge in water is not going to be the object of this study. The purpose will be to determine the relation between the average water evaporation rate on the average electric heating power during the electrolysis. We want to either confirm or to contradict that experimental points are located above the reference line established with the ohmic heater. How will power measurements be made? I have to think about this. Rapidly fluctuating electric current, and arcing in the liquid, are likely to create problems. Please write to me, if you have some suggestions.
11) Be aware that experimenting with electric equipment, especially with a high voltage (and high power) supply, can be fatal if safety precautions are not taken. I will assume you have the necessary equipment and experience. Those who are interested should contact me in private. Let me end this invitation with a message posted by James Mackey, in another context. According to Bridgeman “[The scientist] cannot permit himself any preconception as to what sort of results he will get, nor must he allow himself to be influenced by wishful thinking or any personal bias” This seems wrong. How can one devise an experiment without having some preconceptions about the results expected. How does one decide what to measure without preconceptions about the results. The problem would arise when one allows “wishful thinking” to bias his conclusions based on the experimental results. Everyone has preconceptions about his experiments, so saying that a scientists cannot permit himself any preconception is wrong.
12) My prediction -- call it a preconception or an educated guess, if you wish -- is that the experimental data of Pierre and his colleagues will be confirmed. I also predict that the excessive heat will eventually be explained as due to well known chemical reactions. On the other hand, I remain open-minded toward other explanations. Japanese scientists, like Mizuno, whose experiments were replicated in Paris, are highly qualified chemists. They also think that excess heat released during the electrolysis is too large to be due to chemical reactions. Furthermore, they presented evidence of transmutation of chemical elements. That seems to point toward nuclear origin of excess heat. The controversy about this is not new. Do not miss an opportunity to expose students to meaningful research. They will benefit from working on this excess energy project no matter what the final verdict will be.
Appended on 8/31/05:
What I would like to do, if at least four or five teachers make tentative commitments, is to create a dedicated private discussion list. Informing each
other about progress, and addressing problems, should be easier on a discussion list than via regular e-mail messages. I know that many on this list have
the necessary experience but no time to perform experiments. Such people are also invited; we will need advisers. Please indicate, in a private message
to me, what role do you want to play -- that of a researcher or that of an advisor?
Appended on 9/7/05:
I did not realize this in august but it is now obvious that the French setup is suitable for a student lab in physics or chemistry. No special electrodes
or high voltage power supply is needed -- only ordinary water and an immersion heater. By using the setup of figure 1
(with an ohmic heater only) stdents will collect data to trace the pink curve (see figure 2). Then they will turn the electric current off and collect
the temperature-versus-time data. From that curve they will determine the amount of thermal energy lost in 5 minutes due to conduction, convection and
radiation. Assuming heat losses by these three mechanisms near 95 C are not very different from those at 100 C, and knowing the heat loses by all four
mechanisms, students will be able to show that L is indeed close to 2260 J per gram.
Here is a numerical illustration based of figure 2: (p=400 W time=5 min, energy=400*5*60=120,000 J). The amount of water evaporated is 36 grams. But
the energy used to evaporate that amount is less than 120,000 J because only part of electric energy was used on evaporation. Suppose we know (from the
analysis of the cooling curve near 95 C) that 40,000 J is lost in 5 minutes via three other mechanisms. Then
L = (120,000-40,000)/36 = 2222 J per gram
By the way, this might also be a good project for the annual aapt apparatus competition.
Appended on 9/12/05:
Three days ago the following reply to my announcement appeared at the Physl-L list for physics teachers:
“ . . . and I suddenly realized this would make a capital demonstration of the generation of energy from thin air. One
would immerse a pressed metal powder cube in water, heat it to 95 deg C and then monitor its cooling rate. Then, using the sintered powder as an
electrode, one would impregnate the cube with a gas, say hydrogen, by electrolysis and repeat the cooling rate observations. The cube , now provided
with internal thermal impedance, would cool at a different rate - one supposes, more slowly. If this were the case, how easy to argue that the cube
was demonstrating internal heat production.”
Responding to the above I wrote: “
1) I had in mind is a common immersion coffee heater (or something like that).
2) Nowhere did I suggest a "pressed metal powder cube in water."
3) But I appreciate mentioning a scenario to be avoided. The idea that the so-called "excess heat" might be nothing more than
previously accumulated energy is certainly valid. That issue must be addressed in any serious investigation.
4) How many joules of thermal energy could be injected into water from your 1 cm3 cube, Brian? How does it
compare with 100 kJ of excess heat, presumably measured in France.
5) Please note that two different experiments were suggested:
a) measuring latent heat L of boiling water at 100 C (checking that it is the same as L of evaporation).
b) testing an "excess energy" claim made in the French paper.
6) My tentative expectation (based on two experiments performed so far) is that the excess heat is apparent; it is not nuclear. But I remain
open-minded.
7) Is it reasonable to think that the amount of energy needed to boil one gram of water, near arcing and sparking electrodes (very high local T),
is significantly less than L at 100 C? That would be an explanation of French results.
8) In my opinion students would benefit from addressing this claim, as explain in:
http://blake.montclair.edu/~kowalskil/cf/252clauzon.html
In my opinion the topic does deserve discussion on this list. Why do we prefer to discuss things about which most of us know very little? Take advantage
of a real claim (made by real scientists) that can be made meaningful in a course you are teaching. Promote critical thinking and creativity. Your
students deserve this.
9) I am going to work on that "excess energy" project at the end of this month. Instead of working alone I will be working with a man who
has recently built MOAC. That stands for Mother Of All Calorimeters. His prediction is that even experimental data reported in the French paper will not
be confirmed. PLEAS READ my unit #252 (the URL above)
and join us, if you can.
10) Give your students a chance to be involved in an ongoing controversy. What kind of harm can possibly result from this? Please reply ! ! !
Nobody answered my question about the amount of energy that can be released from a pressed metal powder cube. But one teacher found a student interested in the aspect (a) of the project. As it stands now, two groups will be working on the aspect (b) of the project (trying to replicate the French experiment), one team will be working on the aspect (a) of the project (measuring L), and one person will investigate reliability of electric energy meters when currents are highly irregular. Arcing and sparking does involve irregular currents and an investigation focusing on this aspect of the excess energy study is likely to be very important. Instead of working on the aspect (b) alone I am going to work with two researchers in Austin, Texas. At least one of them is much more qualified than I am in the areas accurate measurements and advanced instrumentation. The other team trying to replicate the French experiment consists a university professor with two students.