A Short History of Laboratory Robotics
Kevin Olsen
Chemistry and Biochemistry Support Staff
Montclair State University
Upper Montclair, NJ, 07043
Almost as soon as electrical instruments became available at the end of the 1800s, some researchers began constructing automated measurement systems. During the 1930's and 1940's a small number of crude automated devices were introduced in the chemistry laboratories. The labor shortages and demanding production timetables of the Second World War provided additional incentives. By the 1950's scientists were beginning to envision how laboratory processes could be automated. The invention of the transistor and the widespread availability of off-the-shelf electronic components allowed more individual scientists to enter the field.
Until the 1980s however, there was no major supplier of laboratory robots. The Zymark Corporation of Hopkinton, Massachusetts was founded specifically to bring turnkey robotics and automation to the scientist. They also were willing to integrate into their systems the growing number of automated devices being introduced by the chemical instrument companies.

An example of a Zymark Pi system. This type of system was known as a Pi system because the components are arranged in circle. The robot in the center passes consumables and samples from one workstation to the next. Courtesy Caliper Life Sciences, formerly Zymark.
Zymark was established by a group of scientists led by Frank Zenie. Because they were designed to work with small objects such as test tubes and pharmaceutical tablets, Zymark's robots did not have to be powerful machines. They were thus safer for inexperienced users and most were small enough to fit into the average laboratory.
In addition to building over 2,500 robotic systems, Zymark promoted them through a dedicated sales force and by sponsoring the ISLAR symposium. (International Symposium for Laboratory Robotics and Automation) Between 1983 and 2003, hundreds of scientists from around the world would gather in Boston to discuss the latest advances in laboratory automation. It is to Zymarks great credit that the symposium was far more than a mere showcase for its sponsors products. The founders of Zymark were scientists who understood the importance of open communication. Thus, many of the presentations featured competing systems and alternative technologies. Some scientists believe the ISLAR symposium, Zymarks promotional efforts, and the large number of scientists who once worked for the company, essentially created the laboratory automation industry.
Zymark's success encouraged others to enter the field. Some entrants were start up companies that were founded specifically to produce laboratory robotics. Others were industrial robotics manufacturers who saw a new opportunity. More and more established instrument corporations entered the market by introducing automated versions of their existing products.

The BIO-6 system is an example of how an industrial robot can be adapted for laboratory use. The arm has six degrees of freedom which allows easy loading of samples into a microscope. Courtesy SSI Robotics,
The American Chemical Society created the Laboratory Robotics Interest Group (LRIG). The growth of e-mail and the internet allowed users to communicate and share ideas much more rapidly. Membership grew quickly and today the group numbers more than 20,000 scientists in over 200 countries. In the United States the group has chapters in the Mid Atlantic, New England, upstate New York, the Chesapeake region, the southeast, Midwest, and the west coast. The Association for Laboratory Automation (ALA) was founded in 1996. Today the ALA has 1000 members and serves automation professionals in all scientific fields. They publish the Journal of Laboratory Automation and sponsor an annual conference with about 4000 attendees annually. Over 400 speaker abstracts were submitted for their most recent conference.
The time was also right for similar growth in the pharmaceutical industry. Towards the end of the 1980s and into the 1990s the pharmaceutical industry was entering its greatest period of profitability and political power. The former financed the robots and the latter enabled the industry to push for FDA acceptance.
The pharmaceutical companies were also desperately in need of labor-saving devices. The size and complexity of initial drug discovery was increasing. By the mid-1990s a typical drug discovery project might involve testing several tens of thousands of drug-like compounds against a disease target. By the end of the decade, projects with 100,000 or more compounds were considered small. The only way test this number of compounds within a reasonable time frame was first to miniaturize the assay and then find ways of automating it. Fortunately, much of the technology was already used in the medical diagnostics field.

The first microplates used for laboratory automation had 96 wells. Each individual well contains enough reagents to perform exactly one chemical or biological test. The microplate is designed to be gripped by a robot. By running tests in parallel, the throughput of an assay is vastly increased. This plate was manufactured by Corning Costar and the bar code label was printed and applied with a device manufactured by Artel Inc. Courtesy Artel.

The 384-well microplate was the next step up from the 96-well plate. Today, 1536-well plates are also widely used Courtesy Labcyte, Sunnyvale Ca.

In this photograph a robotic gripper presents a group of plates to a bar code reader. Courtesy Matrical.
The process of adapting the medical diagnostics technology to automated drug discovery and then creating new versions of it, resulted in a new scientific discipline known as High Throughput Screening, or HTS. HTS Systems cut the initial phase of drug discovery from several years to several months. Because the cost of developing a new drug from initial discovery to marketed product had reached 2,000$ a minute, HTS quickly found wide acceptance among pharmaceutical companies.
The Society for Bimolecular Screening emerged as an important professional organization within the HTS community. They have pressed for the adaptation of standardized dimensions for laboratory microplates. This has vastly simplified programming tasks as have their push for uniform standards for passing data and commands between different types of automated devices. They have promoted the development of the field with the Journal of Biomolecular Screening.

A typical HTS system for use with microplates. Note how the robotic arm is mounted on a track that allows it to move microplates from workstation to workstation. Courtesy The National Institute of Allergy and Infectious Diseases.
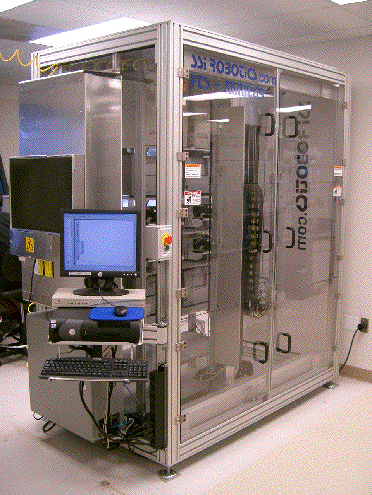
SSI Robotics has created an HTS system that is enclosed in its own clean room. Once the microplates and other consumables are loaded, the system can run unattended for up to 24 hours. The robot arm is adapted from an industrial system and is designed to move materials vertically. Courtesy SSI Robotics
At the other end of the process, manufacturing volumes were climbing ever higher. The industry strained to meet the needs of an aging population and the demands of just-in-time inventory systems. Robots in the quality control laboratories began testing drug products for purity, dissolution characteristics, and potency.

This early Zymate system performed USP basket type dissolution testing including changing the baskets automatically. Courtesy Caliper Life Sciences, formerly Zymark.

A dedicated dissolution workstation for USP paddle type dissolution testing. Courtesy Bioanalytical Systems, Inc.

The Zymark Benchmate, equipped with a gripper, balance, filters, liquid dispensers, a balance and optional grinder, could perform a wide range of tasks in the laboratory. Many were purchased by the pharmaceutical industry for tablet testing but others are found in the food and chemical industries. Courtesy GCMS Service.

This JKEM workstation is an X-Y-Z Gantry type robot designed to perform routine tasks in the laboratory. The system pictured is configured to weigh, dissolve and dilute samples in small vials. The small size and flexibility of these systems had made them popular in many industries. Courtesy JKEM, St. Louis Mo.
Today, pharmaceutical applications dominate the laboratory robotics field. A smaller number of systems are found in the food, biotechnology, chemical, and household product industries.
Whatever their work, laboratory robots have some important differences from their industrial counterparts. Most scientists do not have experience in programming motor movements, motion controls, and feedback sensors. The control computers software is designed to make these processes invisible to the average user.
The relative complexity of their designs created the basic division among laboratory systems. When buying a system, the first choice confronting the user is that of robot or workstation? Workstations can perform only one type of task but with minimal programming and high reliability. True robots (from the Czech word for drudge) can perform a greater range of tasks but involve higher levels of complexity.

There is a continuum between the true
multitasking and completely flexible robot and the simplified workstation.
Few systems are at either extreme. Is this robotic dog a true
robot or a workstation?
The first rule of automation is to simplify
the process. Automating an unnecessarily complex and awkward manual
process will not make a laboratory more efficient.
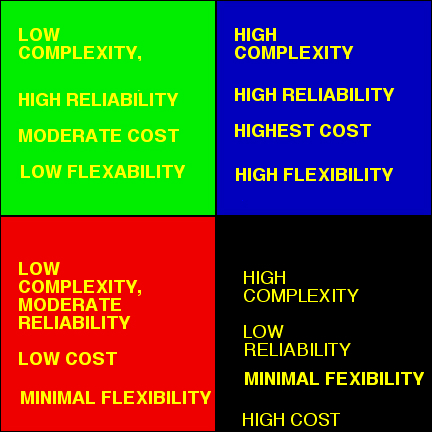
This diagram shows the balance between
complexity, flexibility, cost, and reliability that must be considered when
designing any automated system.
Workstations form the basic building blocks of laboratory automation. The single most common application is liquid handling simply because most laboratory processes take place in liquids, whether analyzing perfume for fragrance molecules, manipulating DNA, or exposing a pathogen to a potential drug molecule. An incredible range of workstations dissolves, dispenses, dilutes, and mixes. The most sophisticated of these can manipulate liquid volumes as small as 0.1 nanoliters. The market for these ultra-low volume devices was expected to be 56 million dollars in 2004 with an annual growth rate of 45%. The total world market for all types of liquid handling workstations has been estimated by Frost and Sullivan to be 500 million dollars. About half of this amount represents medical diagnostics. Pharmaceutical, genomics, and proteomics represent some 75% of the remaining 250 million dollar market. (Robot bartenders are still mercifully in the future.)

In this liquid handling workstation, a group of 96 pipettes moves liquids from one microplate to another. Courtesy Tek Cel and Harvard Medical School.

Liquid handling workstations automate the task of pipetting liquids by hand. Courtesy George Hudson.

A Labcyte dispensing workstation is shown placing 5 nano-liters of liquid into a 384-well microplate. Courtesy Labcyte, Sunnyvale Ca.
Other workstations are conventional laboratory analysis instrumentations that are configured for automated operation. These include spectrophotometric devices, microscopes that can recognize and count different types of cells, mass spectrometers that can analyze several different drug molecules almost simultaneously, and many other types of chemical analyzers. Still other workstations are designed to weigh and dispense powders, incubate live cells, run chemical reactions, or grind samples.
Workstations can operate as stand alone devices. However advances in control software now allow two or more laboratory workstations to be combined into simple automated systems. They can even be equipped with dedicated robotic arms for loading samples.

The Plate Crane is manufactured by the Hudson Control Group of Springfield, New Jersey. Here it is shown loading microplates into a reader. This is a typical use of a robot and automated instrument to create a simple and inexpensive automated system. Courtesy Hudson Controls.
Very large systems can
be created using combinations of multiple workstations. Most robotic arms
found in the laboratory are used to move objects between a systems workstations.
A chemical research laboratory for example, can create a system where one
group of workstations helps synthesize the chemical, another set purifies
it, and the third analyses it for purity. The entire process can be completely
automated by using a robotic arm to pass materials and samples between the
workstations. Similar systems running 24 hours a day are common in drug discovery.
But it is rare for a laboratory robotic arm to manipulate the samples directly,
unlike their industrial counterparts that are often fitted out with welding
tools, paint sprayers, or cutting devices.
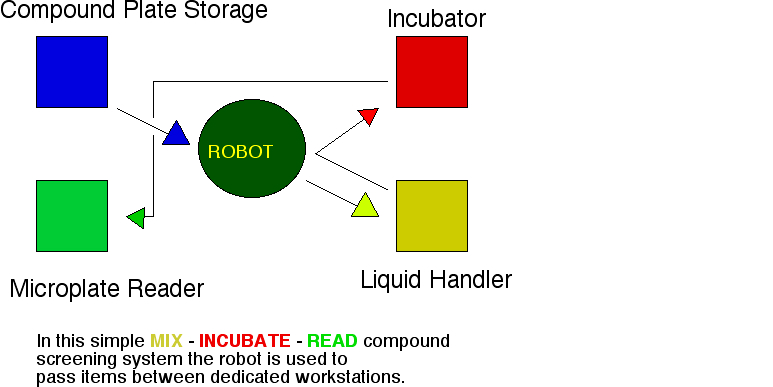
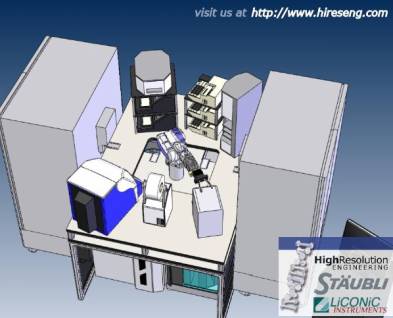
As an alternative to tracked robot arms, some systems use a central arm to move microplates between the workstations. Courtesy High Resolution Engineering

In this system, the two large liquid handling workstations are serviced by a centrally located arm. Transit time is decreased because everything is close together. Additionally, all of the system control components, wiring, and plumbing can be located under the bench. Courtesy Thermo Electron, Burlington, Ontario
The size of the laboratory automation market means that there is no such thing as a "typical" robot user. On one extreme there are many who simply love the technological challenge of running automated systems. They are often found building special components, writing their own software, or rebuilding existing systems for new jobs. On the other extreme are users could care less about all that and just want the robot to run. Robot users come in all ages. If they have any one thing in common it is a deep commitment to doing hands-on science. Women are represented among users in about the same proportion as they are found in the sciences generally. At least a third of all "advanced" users are women.
The laboratory robotics field continues to grow with New Jersey being a major center of innovation. The LRIG would like to invite any scientist, engineer, or student to attend their free meetings and learn about this exciting field. See http://lab-robotics.org